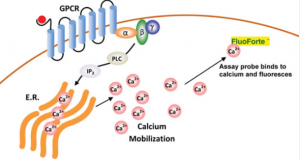
Calcium flux assays measure intracellular calcium ion changes caused by activation of G-protein-coupled receptors or calcium channels, using the membrane-permeable Ca2+ indicator apoaequorin to detect mobilisation within cells.
Entering the World of Calcium Flux Assay Kits
Diving into the realm of scientific discovery, our focus shifts to a pivotal tool in cellular biology: calcium flux assay kits. These kits serve as a window into the dynamic changes in calcium levels within cells, a process critical for understanding numerous physiological and pathological events. Calcium flux assays stand at the forefront of research, enabling scientists to elucidate the intricate mechanisms underlying cell signalling, muscle contraction, neurotransmitter release, and more.
This UK-based platform is dedicated to shedding light on the latest advancements, applications, and technical nuances related to calcium flux assay kits. By exploring the versatile uses of these assays in drug discovery, toxicology, and basic research, we aim to provide a rich source of information and insights. Whether you’re a seasoned researcher or new to the field, our discussions on the technical aspects, experimental design, and interpretation of results from calcium flux assays will guide you through the complexities of cellular calcium dynamics.
Application
Calcium assay kits measure intracellular calcium mobilisation caused by activation or inhibition of G protein-coupled receptors (GPCRs) and calcium channels, including G protein-coupled receptors for hormone imbalance, drug effects, and more. They use a cell-permeable dye that binds calcium directly, emitting fluorescent signals proportional to how much calcium has been bound. Kits come in multiple formats, from high-throughput screening assays to homogenous detection assays for efficient study of numerous biological processes.
Fluorogenic calcium-binding dye used in cell-based calcium assays has been optimized to ensure superior cell permeability and retention, and its self-quenching effect upon binding to calcium produces an order of magnitude increase in fluorescent signal intensity. Cell-based calcium assays can be conducted using various cell types, making them useful in detecting GPCRs, other calcium channels, or receptors.
Cell-based calcium assays offer high sensitivity and a dynamic range, making them suitable for high-throughput screening (HTS) of small molecules that modulate GPCR or calcium channel activity. Eurofins DiscoverX provides traditional Gq-coupled cell lines as well as frozen GPCR cells ready to use with its calcium assay kits; additionally, there is the Calcium No WashPLUS detection kit, which offers more sensitive measurement of intracellular calcium ion flux by eliminating cell washing steps for greater measurement accuracy.
When using a cell-based calcium assay kit, it is critical to tailor both reagents and instrumentation specifically to the application at hand. Make sure your plate reader and liquid handling system can accommodate the large number of reagents used; additionally, check that both your samples and reagents have been stored correctly to avoid evaporation or other contaminants; microscopically examine cell culture before plating to make sure it has been evenly spread out; clumpy cells tend to respond less predictably than single ones.
For the most accurate results, consider using a cell-based calcium assay kit with integrated reagent injectors and automated imaging for kinetic assays. Agilent BioTek Lionheart FX and Cytation imaging systems support measuring and analyzing calcium flux assays with subsecond resolution and high dynamic range.
How it works
Calcium flux assays measure changes in intracellular calcium due to G-protein coupled receptor activation or calcium channel activation and are commonly used for high-throughput screening purposes to identify signalling through GPCRs as an important target class for drug discovery. They use dyes such as Fura-2 AM, Calbryte 520 AM, and Indo-1 as indicators of calcium mobilisation; such tests can often be run using flow cytometers with either endpoint or kinetic modes for reading results.
Calcium assays offer an efficient method for recording neuronal activity compared to multielectrode array (MEA) approaches; they collect data from all observed cells regardless of their position in the plate and require less cell manipulation, making them more user-friendly for HTS applications. Furthermore, calcium assays can be run using the same plates and reader system as GPCR screening assays.
As such, these assays have many advantages over traditional fluorescent methods; however, there can still be limitations, such as poor plate stability or complex cellular engineering, antibody, or dye validation processes required. Thanks to recent innovations that allow true no-wash assay formats like the BD PBX calcium assay kit to overcome such restrictions, these limitations have now been overcome.
The PBX assay kit is a 96- or 384-well microtiter plate-based homogeneous fluorescence assay that does not require cell washing. Compatible with standard automated systems like FlexStation and FLIPR 3 from Molecular Devices, as well as Hamamatsu FDSS 7000 and CLARIOstar Plus multi-mode plate readers from BioTek Instruments, it requires no cell washing before running the assay.
This assay is easy to set up and requires no washing steps or complex cell titration steps, thereby streamlining its workflow and reducing experimental variability.
The assay utilises a non-ionic reagent, which is quickly taken up by cells via capillary action, leaving behind a calcium indicator. When activated via GPCR activation, this calcium indicator is released within cells and fluorescence increases significantly, providing highly sensitive measurement of agonist-stimulated calcium mobilisation with an excellent signal-to-background ratio and an EC50 value proportional to GPCR agonist affinity for the binding site.
Signalling
G protein-coupled receptors (GPCRs) are an array of membrane proteins involved in cell signalling that can be altered by drugs. A calcium flux assay provides a powerful tool for measuring both agonist-stimulated and antagonist-inhibited signalling through these GPCRs—essential in drug discovery! Molecular Probes’ ion indicators offer bright fluorescent signals to track changes in intracellular calcium concentration.
Calcium assays provide an invaluable way of measuring the functionality of neurons derived from human-induced pluripotent stem cells (iPSCs). Fluctuations in intracellular calcium reflect neuronal activity and can be monitored using various imaging techniques.
The Assay Genie Calcium Flux Ratiometric Kit provides all of the reagents necessary for conducting no-wash ratiometric calcium assays on adherent and non-adherent cells without needing to wash between assays. Available with or without probenecid solution and background masking reagent for background masking purposes, enough reagent is included for up to ten 96-well assays using its adapter syringe for fast setup. Using such a device reduces leakage leakage leakage as well as photobleaching effects associated with manual pipetting that may cause leakage leakage leakage or uneven dye loading that might result from manual pipetting techniques used during assay setup and setup time.
Ratiometric calcium assays offer numerous advantages for use on instrument platforms like FLIPR and FlexStation, including the elimination of artefacts caused by uneven compound addition. Furthermore, their kit is optimised to work effectively on these platforms, with enough reagent provided to make a 1:1 mixture between 520 AM dye and syringe-compatible PowerLoad reagent to make cell loading simpler, further enabling assay performance under conditions with complete serum media for maximum assay performance and compatibility with numerous cell types that would normally prove challenging to load using traditional calcium indicators.
To successfully perform a calcium flux assay, it is vital that cells are evenly dispersed among wells before dye-loading begins. Furthermore, liquid handling system dispensing speed and height must be adjusted so as to not stimulate cells during compound addition; an uneven distribution can occur with too high a dispensing speed, and a lower dispensing height could result in clumpy populations that respond less favourably to agonists or compounds.



